Surgical resection remains a mainstay of treatment for many forms of cancer, and the completeness of tumour resection can have a major influence on patient prognosis. However, identifying the boundary between cancer and normal tissue can be very challenging.
A multidisciplinary research group recently described progress in developing a cancer-targeting optical imaging probe that will enable surgeons to clearly visualise the margins between normal tissue and certain cancers in real time and in the operating room. This article describes the development of this specific imaging probe, while providing a broader introduction to the concepts and building blocks of molecular imaging.
SURGICAL NEED
Although the optical imaging probe described has broader cancer applications, the original focus was on paediatric brain cancers, the most common solid malignancies in children. The primary approach to many paediatric brain cancers remains surgical resection. Despite the availability and use of additional chemotherapy and radiation therapy, the completeness of surgical resection in cancer treatment remains one of the most important prognostic indicators.
This can present a significant challenge to the neurosurgeon, as brain tumour tissue can appear grossly indistinguishable from adjacent normal brain tissue. Surgeons are constantly confronted with the prospect of resecting too much normal tissue, leading to serious neurocognitive deficits, versus leaving residual cancer, which will grow and metastasise.
See Also:
A longstanding dream of the senior neurosurgeon on the team was of ‘painting’ brain cancer to clearly define the difference between normal and abnormal tissue intraoperatively and in real time, thus facilitating complete resection while minimising damage to adjacent normal brain.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataTARGET IDENTIFICATION
Molecular imaging has been defined in a number of ways, but one consistent theme has been visualisation of a specific biological process in an intact living system. This contrasts with ‘traditional’ imaging, where the primary goal is visualisation of anatomy or functional imaging, which usually describes preferential blood flow in response to stimuli.
Potential targets for molecular imaging in an intact biological system can be identified in a sequence starting from the gene, progressing to the transcription of messenger RNA, which is then translated to protein, and then finally expressed as protein function. This intranucleolar to intracellular-to-extracellular progression also describes a progressive increase in the number of targets (from two gene pairs per cell to 100–1,000,000 protein targets per cell to the relatively massive amount of targets representing the actual function of the individual and collective proteins). Not surprisingly, most targets for molecular imaging are proteins and receptors expressed in the cell membrane or extracellular matrix.
The biological basis of our imaging probe was the binding of chlorotoxin (CTX) to matrix metalloproteinase-2 (MMP-2). Chlorotoxin is a small amino acid peptide first purified from the venom of the giant Israeli scorpion Leirus quinquestriatus. CTX has been found to have high binding affinity to MMP-2, a membrane-bound endopeptidase which has been found to be upregulated in the extracelleular matrix of certain brain cancers, including gliomas and medulloblastomas, but not normal brain cells.
A previous attempt to target brain cancer for intraoperative imaging focused on uptake by inflammatory microglia surrounding the tumour. The advantage of the biological targeting system that we have developed is its specificity to the actual tumour cells. In addition, the steroids frequently administered to patients to diminish brain edema inhibit microglial activation but would be expected to have no effect on CTX binding to MMP-2.
PROBE SYNTHESIS AND EVALUATION
Numerous imaging strategies and probes have been developed and described for molecular imaging. Not surprisingly, just as in clinical imaging, no perfect imaging modality exists. Major considerations and trade offs include anatomic resolution, concentration of the probe needed to generate a detectable signal, ease of scanning, radiation dose and tissue penetration.
Optical imaging techniques, such as fluorescence and bioluminescence, have several distinct advantages: imaging equipment and start-up costs are relatively low and a wide range of imaging probes for conjugation to targeting molecules is available. Also the techniques offer the greatest imaging sensitivity: the smallest concentration of delivered probe that can be imaged is 10-15–10-17M/L, as opposed to 10-10-10-12M/L for nuclear medicine imaging and 10-3–10-5M/L for MR contrast detection.
The main obstacle for optical imaging as a translational (or bench to bedside) imaging modality has been poor tissue penetration. Emitted photons are typically absorbed within 1–2cm by body tissues. While not an issue for an experimental mouse that may be only 2cm in total body diameter, deep organ imaging in even an infant can be defeated by a 2cm depth limit. Where the 1–2cm limit does not present a problem is in superficial imaging, such as submucosal imaging of the bowel using endoscopy or in the open surgical field.
Our choice for optical imaging probe or ‘beacon’ was Cy5.5, a fluorescent molecule that emits photons in the near-infrared (NIR) spectrum. The advantages of using fluorescent beacons in the NIR frequency include minimisation of autofluorescence interference from adjacent normal brain, relatively efficient penetration and depth detection of this wavelength, and minimal absorption by water or haemoglobin.
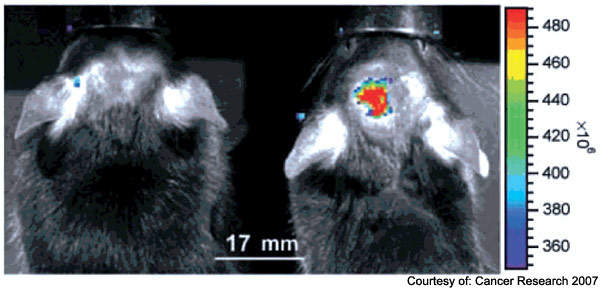
Following synthesis of the probe, multiple in vitro and in vivo experiments were performed to demonstrate tumour-specific binding via MMP-2 targeting, passage through an intact blood brain barrier, biodistribution and toxicity of the probe, and visibility of the probe during surgical procedures performed in genetically engineered mouse models of cancer (Figure 1).
The probe demonstrated exquisite specificity for distinguishing cancer from adjacent normal tissue: all specimens ‘painted’ by the CTX:Cy5.5 signal as cancer were confirmed to be cancer, and all adjacent non-painted tissues were confirmed to be histologically normal.
Equally important was demonstration of passage across the intact blood brain barrier and long duration of probe activity, with brain cancer cells clearly distinguishable from normal tissue at 14 days, enhancing the utility of this probe for surgical application.
Finally, although brain cancer imaging was the initial focus, broader application to other cancers expressing MMP-2, including prostate, gastrointestinal, sarcomas and lymphatic channel metastases of only a few hundred cells, revealed a similar ability of the probe to paint and distinguish cancerous from adjacent normal tissue (Figure 2).
NEXT STEPS
This article briefly summarises our progress to date in realising the vision of intraoperative tumour painting to enable surgeons to distinguish the margins of cancerous from non-cancerous tissue in order to maximise tumour resection while minimising damage to or removal of adjacent normal tissue.
This research represents the combined efforts of many investigators from multiple disciplines, ranging from materials science engineering to molecular tumour biology to surgery and imaging science. The research also required the combined and shared resources of multiple institutions, including the Fred Hutchinson Cancer Research Center, the University of Washington, and the Seattle Children’s Hospital and Regional Medical Center. As such, it truly represents a necessary future model of multi-disciplinary and multi-institutional research to advance the field of molecular imaging.
Related research being performed by this research team includes the development of targeted hybrid nanoparticles to provide MR contrast enhancement for presurgical planning with MR imaging followed by intraoperative fluorescent painting provided by the CTX:Cy5.5 fluorophore.
Furthermore, our group envisions functionalising these probes to carry chemotherapeutic and biotherapeutic payloads for targeted delivery and tumour cell kill, with the goal of maximising cell kill while minimising toxic side effects.
A related area of research interest is the development of monitoring probes to non-invasively and quantitatively detect cell death in response to therapy, resulting in the dramatically truncated follow-up times required to assess efficacy.
Ultimately, one can envision a complete suite or toolbox of cancer customisable molecular imaging probes enabling presurgical detection and planning, intraoperative visualisation and guidance, targeted delivery of cancer therapeutics and non-invasive rapid assessment of therapeutic cell kill.
While very encouraging, this early success in genetically engineered animal models of human cancer represents the beginning of a long road to human application, with multiple trials required to demonstrate safety and efficacy.
Importantly, though, this reseach does represent a significant and important step towards the realisation of the goals of enabling surgeons to clearly see the difference between cancer and non-cancer, dramatically improving the completeness of cancer resection, and ultimately improving the survival and quality of life for the patients that inspire us to undertake this research.







