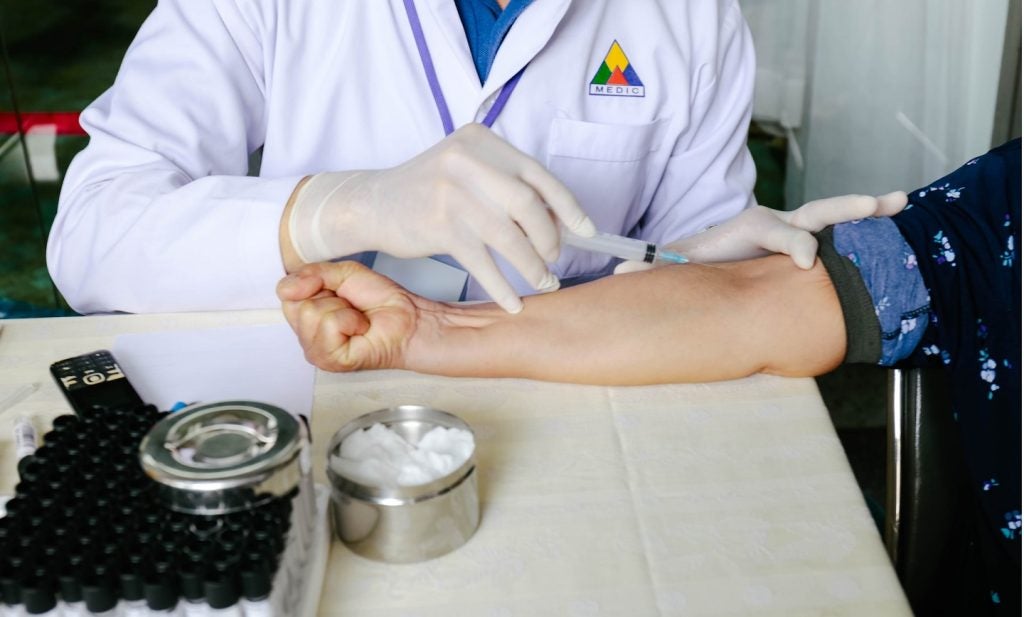
As the race to find a vaccine to quash the swell of H1N1 (swine) influenza cases draws closer to the finish line, governments around the world are left with more questions than answers. First off is who should get the treatment? Previous flu jabs have historically been given to the elderly and those with chronic diseases. The H1N1 strain seems, conversely, to be more dangerous for younger populations. Although common rhetoric assumes that healthcare staff will be at the forefront of inoculations, a recent study by The Nursing Times found that only one in three nurses are prepared to have the vaccine themselves.
Next is the question of how many doses governments should order. The latest statistics from the World Health Organization (WHO) show that Northern Hemisphere countries have so far ordered more than one million doses with Greece, the Netherlands, Israel and Canada ordering enough for their entire populations.
In addition, there are still questions over whether double doses will be needed or if pharmaceutical firms will be able to produce as much of the preventative treatment as they first hoped. Add to that the dreaded question of what happens if the strain mutates further – putting all the drug development companies’ work to waste – and the situation looks a little less certain than many pharmaceutical public relations executives would have us believe.
However, the two biggest questions are whether the vaccine will be safe in the first place and if national healthcare infrastructures have enough capacity to administer all the jabs that will be needed in a mass inoculation programme. Without answers to these questions, all the rest falls into oblivion.
Ensuring vaccine safety
See Also:
In early August, the WHO responded to media reports questioning the safety of vaccines for pandemic influenza with reassurances but it also stressed that getting vaccines developed quickly was of critical importance.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData“Vaccines are among the most important medical interventions for reducing illness and deaths during a pandemic. However, to have the greatest impact, pandemic vaccines need to be available quickly and in large quantities,” the WHO’s statement said. “During the 1957 and 1958 pandemics, vaccines arrived too late to be used as an effective mitigation tool during the more severe phases of the pandemic.”
The WHO also said that regulatory authorities have shown great flexibility in developing procedures for fast-tracking the approval and licensing of pandemic vaccines. To ensure vaccine safety in the US, for example, the National Vaccine Advisory Committee has established the H1N1 Vaccine Safety Subgroup. With the remit of providing feedback on the strength of safety monitoring for a 2009 H1N1 influenza vaccine, it has come back with a number of recommendations.
The guidelines include the need to monitor vaccine recipients for vaccine adverse events, especially where they have been combined with an adjuvant. It also recommends that consideration should be given to a transparent and independent review of vaccine safety data as it accumulates.
Before the safety of a vaccine in the mass population can be ensured, however, it first needs to be developed in a watertight clinical trial. GlaxoSmithKline (GSK) media spokesperson Stephen Rea says that GSK has already begun trialling its vaccine in Germany. In many ways this is similar to any other trial the company has conducted. Rea says a couple of factors make the company particularly well suited to producing the vaccine in a safe manner. The first is that we have made seasonal flu vaccine for a couple of decades now and because of that we are quite confident in the process of developing the vaccine,” he explains. In addition, the technology we are using and the egg-based production method to grow the vaccine is the same.”
Rea says that the first trials are taking place using healthy adults but the full spectrum of the population will be tested to ensure it has minimal side-effects for all age groups. Many questions that have been raised are surrounding the adjuvant being used. For GSK’s H1N1 vaccine the company is using the AS03 adjuvant – an oil and water-based compound – that was also added to the H5N1 vaccine made in preparation of an avarian flu pandemic.
Rea is keen to stress AS03’s safety record and the benefits that can be garnered. “We have used AS03 for quite a long time,” he says. “We have done trials on 40,000 people with it and it has been used globally. It serves two purposes as it primes the immune system which then gives you a greater immunogenicity response. Secondly, it means we can use a lot less of the actual strain so you get a lot more doses.”
GSK will conduct a post-marketing study once ongoing safety is ensured and as more people become exposed to the vaccine. There will be further monitoring by the Medicines and Healthcare products Regulatory Agency, an agency in the UK’s Department of Health.
Hospital capacity
Even with the most stringent safety regulations, without adequate capacity and preparation in a country’s healthcare sector the vaccine still might not be able to reach all of those in need of the inoculation.
Along that line, national health services around the world have begun to turn to the private sector for assistance. India has been proactive in designating certain hospitals to treat H1N1 patients and Saudi Arabia’s government has announced it will bear the cost of treating all influenza patients whether they are in private or public care.
In the UK, the British Medical Association is negotiating what additional funding doctors will receive to carry out swine flu vaccinations, with UK newspaper The Times reporting that the figure could be as much as £300m. The union has been lobbying for the extra cash equalling £6.92 per patient to deliver the inoculation that will be given alongside the seasonal flu jab. But the action has provoked widespread criticism, with many stating it is a general practitioner’s duty to provide frontline care.
Many countries are expecting delivery to begin in October 2009, missing the start of the school season. Nevertheless, it is children that stand at the forefront of the inoculation programme. Alongside this group are childcare providers and healthcare staff.
With clinical trial results not yet released there are still many unknowns and emergency capacity planning around the world is being carried out based on a series of guesses. Until they know the facts about just how many doses pharmaceutical firms will be able to produce in the first few months of a vaccine being released, there is only so much hospitals, doctor’s surgeries and governments can do.
In the meantime, rigorous standards are being set up with, for example, the UAE’s National Supervisory Committee for Combating Swine Flu warning that legal action will be taken against any hospital – public or private – that violates the government’s directives in dealing with the disease. Violations include negligence towards any patient with swine flu symptoms and inspection teams have been set up to monitor whether standards are being met.
So while some are working around the clock all others can do is put in place preparation procedures until the real work can be done. In the meantime, antivirals are still being distributed, national hotlines set up and hopes pinned on the virus not mutating further or becoming more virulent in the future.