Tumours, bone cysts and traumas, as well as osteomyelitis and osteolysis, can cause massive bone loss. The primary method used today for filling such defects is autogenic or allogeneic bone transplantation. As both approaches are associated with considerable problems there is an ongoing critical debate as to whether these procedures should still be considered the treatments of choice1,2 Autogenic bone transplantation, for instance, is always associated with another surgery.
For the patient, the surgical removal of graft material means additional stress and a higher postoperative morbidity due to local complications.3 In addition, the amount of graft material that can be harvested from the patient’s own body is limited. Economic analyses provide additional arguments for challenging the use of autogenic bone material. Given the extended duration of surgery, the use of autogenic bone grafts involves higher costs. Although allogeneic bone transplantats have the advantage of higher availability, their inherent antigenicity is a major disadvantage.2
BENEFITS OF BONE SUBSTITUTES
Given the multitude of problems associated with autogenic and allogeneic bone transplantation, the search for bone substitutes similar to bone grafts in terms of biomechanical strength and biological properties has intensified over the past few years.
Such materials should support the bone healing process and ideally should be associated with osteoconductive and osteoinductive properties and biocompatibility. Apart from natural materials obtained from corals or algae, there are inorganic materials such as ceramics made of hydroxyapatite or tricalcium phosphate. Other materials include xenografts and glass ionomer cements.
See Also:
BIPHASIC CERAMICS
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
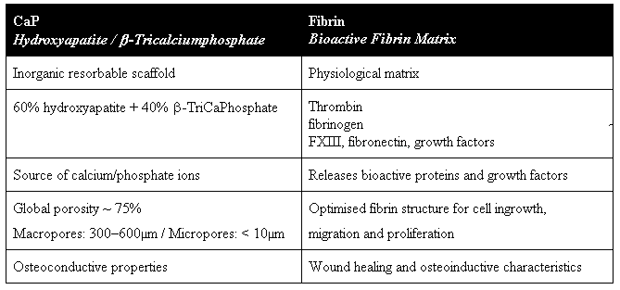
By GlobalDataThe development of Tricos® represents real progress in the field of bone substitutes. The idea behind this bone substitute was to ensure structured, physiological formation of bone substance. The biphasic ceramic containing hydroxyapatite (HA, 60%) and beta-tricalcium phosphate (ß-TCP, 40%), fulfills these requirements in several ways (see Table 1). This is used in combination with a fibrin matrix made of human plasma.
In the new material there is a balanced equilibrium between the quickly resorbable beta-tricalcium phosphate and the slowly resorbable hydroxyapatite.4,5 ß-TCP has a high osteogenetic potential and constitutes a source of P+ and Ca++ ions.6
Simultaneously, with the progressive biodegradation of ß-TCP, biological apatite crystals begin to form, gradually replacing the bone substitute analogously to natural remodelling.
Hydroxyapatite guarantees the long-term stability of the material; it ensures that the bone substitute supports cell adhesion in the long run, acting as a carrier. In its chemical structure the substance resembles the bone’s biological apatite crystals, and it is only mildly osteogenic.
The new bone substitute has an interconnective pore system consisting of micropores and macropores; the granules are 1–2mm in size (see Figure 1). The micropores less than 10µm in size provide for a rapid exchange of fluid and ions by means of diffusion. With their size of 300µm–600µm, macropores permit the adhesion and proliferation of osteogenetic cells. This ensures that the bone substitute material is successively replaced, by cells spreading from the periphery to the middle of the ceramic material (see Figure 2).
FIBRIN MATRIX
In order to optimise physiological bone regeneration the biological two-component matrix of Tissucol/Tisseel® is added to the inorganic material. The two-component matrix consists of fibrinogen, plasmafibronectin, factor XIII and plasminogen as well as the plasmin inhibitor aprotinin.7 The human thrombin contained as a second component triggers the final step of physiological blood clotting, the conversion of fibrinogen to fibrin.
The completely resorbable fibrin is used in all surgical fields. The fibrin matrix penetrates both micropores and macropores, and provides high stability through adhesion to the walls of the bone defect. Simultaneously, the fibrin matrix serves as a binding agent for the granules. Its content of polymerised fibrin, fibronectin and growth factors helps trigger cell proliferation and angiogenesis, and enhances wound healing.8,9
On a cellular level the artificial bone induces signals similar to physiological material. Diffusion of biological fluids into the ceramic´s micropores and macropores triggers the release of calcium ions; macrophages, mesenchymal stem cells, osteoblasts and osteoclasts will then enter the ceramic´s macropores.4
Cell adhesion is followed by cell proliferation, and gradually, osteoclasts will resorb the so-called artificial bone in a process similar to physiological remodelling, while osteoblasts will build new bone material.
Application of Tricos is simple and straightforward. The preparation is ready for application approximately two minutes after mixing the two components. Similar to a plastic glue, the mixture can be applied with a spatula and used for anatomical reconstruction.
PRECLINICAL AND CLINICAL DATA
The benefits of biphasic ceramics combined with a fibrin matrix have been shown in many preclinical and clinical trials over the past few years.6,10–15 The easy-to-use, pliable material allows successful anatomical reconstruction, proving both biocompatible and stable.
Histological examinations reveal the polymerised fibrin to have a stable structure on which ions crystallize, thus enhancing intrinsic osteogenic properties. Biopsis shows that the material is progressively replaced by new lamellar bone within two or three months. A retrospective long-term trial over 16 years supported the material´s long-term tolerability, good bioactivity and high osteointegration rate.
SUCCESSFUL TREATMENT TRAIL
At Würzburg Orthopaedic University Clinic further comprehensive clinical data on Tricos was collected between 10/2004–08/2005. During a one-year clinical trial, bone defects in a total of 17 patients aged between ten and 69 were treated using the new bone substitute. The material was used for filling various bone defects caused by benign tumours or tumour-like lesions.
There were five patients with benign bone tumours (enchondrome, chondromyxoid fibroma, chondroblastoma, osteoma, osteoblastoma, osteoclastoma) and 12 with tumour-like lesions (juvenile bone cyst, non-ossifying fibroma, eosinophilic granuloma, intraosseous ganglion, fibrous dysplasia). The cysts were surgically removed and each bone defect then filled with two to ten syringes containing 3.5cm3 of Tricos, i.e. 7–35cm3 in total.
During this one-year trial the bone substitute proved both easy to use and safe. There were no complications. The long-term data after nine months showed that the biomaterial had successfully integrated into the bone; resorption was not yet complete.
REFERENCES
- Rueger JM et al. ‘Knochenersatzmittel’.Der Orthopäde 1998; 27:72–79.
- Stützle H et al. ‘Knochenneubildung durch Knochenersatzmaterialien’
.Der Orthopäde 1998; 27: 118–125. - Dütting A et al. Z. Orthop. 1998; 126:44–47.
- Daculsi G et al. Journal of Materials Science: Materials in Medicine 2003; 14:195–200.
- Arinzeh L.T. et al. Biomaterials 2005; 26:3631–3638.
- Daculsi G et al. ‘Macroporous biphasic calcium phosphate efficiency in masoid cavity obliteration: experimental and clinical findings’. Ann Otol Rhinol Laryngol 1992; 101:669–74.
- Guéhennec et al. European Cells and Materials 2004; 8:1–11
- Clark RAF. ‘Fibrin and wound healing’. Ann NY Acad Sci 2001; 936:355–67
- Amram DL. ‘Wound healing. role of commercial fibrin sealants’. Ann NY Acad Sci 2001; 936:566–79
- Daculsi G et al. Journal of Biomedical Materials Research 1990; 24:379–96
- Cavagna R et al. Journal of Long-Term Effects of Medical Implants 1999: 9(4):403–12
- Passuti N et al. Orthopaedics 1989; 248:169–176
- Bertrand B et al. Larygoscope 1996; 106:652–657
- Le Guéhennec L et al. Journal of Material Science: Materials in Medicine 2005; 16:29–35
- Bagot D´Arc. Journal of Materials Science: Material in Medicine 2004; 14:229–33