Acute stroke is a major socioeconomic burden and the development of efficient drugs to combat the disease presents a major challenge to the pharmaceutical industry.
Disabilities such as hemiparesis, the inability to speak and partial blindness, rapidly become irreversible within minutes to hours of the disease’s onset. Prompt, accurate diagnosis by neuroimaging followed by efficient individualised therapy is therefore imperative.
CURRENT TREATMENT
RtPA, a drug that dissolves the blood clot (thrombolysis), is the only established treatment. It is approved for treatment within three hours of the onset of symptoms at highly specialised centres, as there is a risk of bleeding in the brain.
However, this three-hour time window precludes the treatment of patients where it is unclear when the symptoms started. Many patients wake up with symptoms and others do not arrive at a hospital within the required timeframe, which means they cannot be treated.
See Also:
CT is the most widely used imaging technology in clinical management of acute stroke. For example, CT is very sensitive to haemorrhagic stroke (ruptured vessels), which is essential, as the treatment of this small fraction of stroke patients with rtPA will cause severe side effects.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataTOWARDS GREATER PRECISION
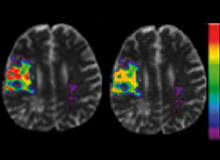
MRI is a growing tool in acute stroke treatment. Research has shown that it can delineate micro-structural damage after acute stroke by using diffusion weighted imaging (DWI) to show regions of cellular damage and measure the reduction in blood supply by perfusion weighted imaging (PWI).
More importantly, MRI has high specificity in predicting the final outcome in acute stroke patients, facilitating the development of powerful systems for the support of patient management and the study of drug efficacy.
Currently, the logistics of performing acute MRI and the lack of access to advanced processing tools and imaging expertise limit healthcare professionals when it comes to accessing the benefits of acute MRI images.
EFFICIENT CLINICAL TRIALS
New drugs that either reduce the vulnerability of brain tissue to the lack of nutrients or allow thrombolysis after three hours are currently undergoing phase II and III trials. One example is desmoteplase, a thrombolytic agent that appears to be safe and efficient in the three- to nine-hour time window.
Such trials are extremely costly and time consuming, as they involve administering the drug to many hundreds of patients in order to prove the beneficial effects of the drug by comparing disabilities and deaths in treated and untreated patients.
It has recently been proposed that drug efficacy could be proved by much more sensitive markers than patient disability. Where the disability depends heavily on the size and location of the tissue damaged by the stroke, and thus requires many patients to demonstrate the drug’s effect on this uncertain area, observing the fate of well-characterised tissue elements through neuroimaging may allow the efficient detection of a treatment signal.
Disease progression models (DPMs) have been developed to predict the risk of subsequent tissue damage. Based on a database of example cases (initial risk factor images combined with final outcome images, showing whether given areas were eventually damaged), they allow healthcare professionals to predict outcomes in new cases with a high degree of accuracy.
By subsequently comparing infarct volumes in rtPA-treated patients with their predicted volumes based on untreated patients, the beneficial effects of rtPA could be shown in as few as 29 patients by MRI and DPMs.
The development of neuroinformatics technology is believed to improve the cost-effectiveness of future phase II trials in general – and provide data for future trials.
IMPROVING THE PROGNOSIS
The technology to provide end-users of new drugs with unparalleled diagnostic support through access to pre-market image data will open up new avenues in industry-driven therapeutic support.
Advanced software to analyse image data from acute CT or MRI scans is already in high demand. An easy-to-use DPM model based on untreated patients, which would allow even small hospitals with limited expertise to carry out diagnostic imaging with computerised support based on numerous, well-diagnosed cases, is believed to have huge market potential. There is also the additional option to market drug-specific DPMs for individualised treatment within the pharmaceutical industry.
The pharmaceutical industry and academic biomedical researchers are working hard to identify drugs that will stop tissue damage progressing hours and days after acute stroke. Hundreds of promising candidates have been identified and observed to limit brain damage in animal studies, and dozens of therapeutic approaches are currently undergoing trials in humans.
This quest, however, faces a number of problems. First, drugs with beneficial effects in animal models of stroke have shown disappointing effects in humans. Secondly, the efficacy of drugs tested on humans is typically determined by ‘weak’ indicators, such as neurological scores or the final volume of damaged tissue. Both approaches require hundreds of treated and untreated patients, rendering drug trials lengthy and expensive.
The past decades of intensive and expanding stroke-related research have yielded enormous data resources on the nervous system, ranging from genomic to behavioural levels. Collectively, these data will help to disclose disease mechanisms and target the development of efficacious therapeutic approaches.
Two decades ago, through the introduction of bioinformatics, molecular biology passed beyond the manual stage of scientific practice that neuroscience is only now beginning to leave behind.
THE FUTURE OF NEUROSCIENCE
The first prediction system to be tested in clinics is the knowledge discovery tool I-Know. It is also the first to utilise genetic, epidemiological, clinical and biochemical parameters in predictions.
The research partners have explored new instance-based data mining techniques to improve predictive power. Using sophisticated supervised learning algorithms such as the MARS approach, they have developed methods to quantify the ability of each of the MRI-derived physiological variables to predict tissue death – that is, by using knowledge from discovery-based disease models they can infer quantitative pathophysiological information.
I-Know is the first attempt to use stroke progression models to address the pathophysiology of stroke by identifying and quantifying the role of genetics, epidemiology and other clinical features in stroke progression.
The researchers determined the predicted risk of infarction in untreated and treated patients (n=12 untreated, n=29 treated by standard i.v. rtPA thrombolysis). By comparing this with actual final infarct volumes, they were able to statistically demonstrate that treatment significantly reduced the predicted final infarct size.
This is a significant finding, as the number of patients in this study is a factor of 100 less than those who have until now gone into studies proving the clinical effect of iv-rtPA and a factor of 10 less than demonstrating drug efficacy by imaging infarct size.
This shows a definite role for neuroinformatics approaches in identifying disease mechanisms in multi-modal data and accelerating drug development by the early identification of active drugs.
I-Know will be the first tool to use data mining to test the efficacy of new drug candidates in phase II of drug development. It therefore offers a unique opportunity to exploit this leading role within drug development and diagnostic prediction systems for the benefit of Europe’s industry as well as its citizens.
Cerebral blood flow (CBF) is traditionally used as an index of brain function and to detect impaired blood supply or cerebrovascular reserve capacity. Normal values in grey matter range from 45ml to 60ml of blood per 100ml of tissue per minute (resting values display a steady decrease with age), with pronounced regional increase in response to functional activation or pharmacological perturbation (such as CO2 or acetazolamide).
It is traditionally believed that a CBF of at least 18ml/100ml/min is required to supply sufficient oxygen for cortical electric activity, while a CBF below 12ml/100ml/min results in neuronal death. White matter flow rates are relatively constant at approximately 20ml/100ml/min, with no noticeable decrease with age.
Thrombolysis requires fast and effective imaging to rule out extensive infarction and haemorrhage, preferably detecting the exact size and location of the infarct. Major stroke trials (NINDS and ECASS) have used conventional CT scans for patient selection. CT, however, has demonstrated low sensitivity to acute cerebral ischemia – approximately 75% during the first six hours.
Therapy is therefore based on clinical presentation, the time of onset and negative findings on a conventional CT scan. Evidence is accumulating that identification of an ischemic core and the ischemic penumbra by MRI improves patient outcome. MRI is now increasingly used in acute stroke.
Diffusion weighted imaging demarks infarcted tissue as early as minutes after the onset of symptoms, while T2* weighted MRI is at least as sensitive as CT in detecting cerebral haemorrhaging.
Perfusion weighted imaging (PWI) delineates areas with altered blood flow, and at high risk of infarction, known as the penumbra. Combining stroke MRI modalities thus allows the final infarct size to be predicted. The aim of thrombolysis is to save ischamic yet viable cerebral parenchyma.
In contrast, extensive infarcts or infarcts with little or no penumbra have an unfavourable risk-benefit ratio. Combined perfusion and diffusion imaging therefore provides important information and has been suggested as a prominent selection criterion for thrombolysis in clinical decision-making.
Recent studies suggest that using MRI for a selection of patients with thrombolysis increases the chance of a favourable outcome, even in an expanded timeframe. Efforts remain, however, to identify patient sub-groups (by criteria including symptom duration, clinical severity, and infarct size and location) in which MRI may successfully expand the use of rtPA.
Despite the advantages of MRI, this modality is not feasible in all patients (such as patients with metal implants, pacemakers or unstable clinical conditions) and there is a pressing need to establish whether inherently longer MR scan times delay treatment.
It is clear that applying new technologies to the treatment of stroke victims is improving diagnosis and treatment, leading to better recovery rates. New developments in clinical trials are also showing great promise, allowing drugs to be tested more efficiently so that they can reach patients faster.