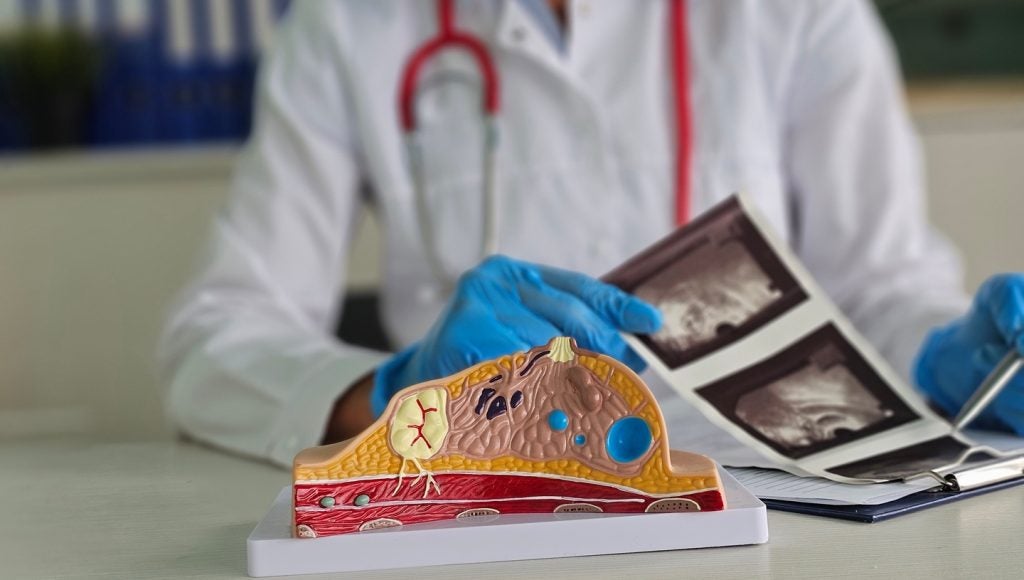
BD (Becton, Dickinson and Company) has received 510(k) approval from the US Food and Drug Administration (FDA) for the EnCor EnCompass breast biopsy and tissue removal system.
The approval marks the introduction of a multi-modality system designed to provide clinicians with flexibility across breast imaging modalities for diagnosing breast disease.
The system aims to streamline breast biopsy procedures by supporting use across various breast imaging platforms within one integrated device.BD expects to bring the system to market in early 2026, highlighting features designed for procedural efficiency.
Key technical specifications include a variable sample notch adjustable during procedures, compatibility with different imaging platforms, high and low vacuum strengths, an echogenic cutting cannula for visualisation, 360° sampling capability, probe options of 10G, 7G, and 12G for various lesion types, and an illuminated sample container.
The FDA clearance broadens BD’s range of breast health technologies and supports its ongoing efforts in early detection and diagnosis.
BD peripheral intervention worldwide president Rima Alameddine said: “This milestone for our new breast biopsy system marks a meaningful advancement in breast health, playing a critical role in aiding the early detection and diagnosis of breast disease.
“This innovation underscores our commitment to partnering with clinical leaders to deliver patient-centred solutions. Guided by our vision to transform breast health, we remain focused on developing technologies that empower providers and inspire confidence in care.”
BD interventional–peripheral intervention oncology platform vice president and general manager Stacie Watson said: “The FDA clearance of the EnCor EnCompass Biopsy System demonstrates our ongoing focus on addressing the evolving needs of clinicians and patients in breast health.
“This multi-modality platform is engineered to provide flexibility, control, and ease of use, with features designed to support both clinician confidence and the patient experience.”
In August 2025, BD announced an investment of more than $35m to expand the manufacturing capacity for BD PosiFlush prefilled flush syringes at its facility in Columbus, Nebraska, US.