Cappella is a medical device company that develops novel solutions for the treatment of complex coronary artery disease (CAD) and specifically bifurcation vascular disease. Cappella’s initial product, the Sideguard® Coronary Sidebranch Stent and Delivery System, offers interventional cardiologists a straightforward, effective solution that focuses on treating the sidebranch of diseased coronary arteries first, rather than the main vessel.
More importantly, it allows the preferred stent of choice for the main vessel. An optimal stent design specific to the anatomy of the sidebranch, combined with the qualities of nitinol, now provides a dynamic solution for treating sidebranch disease.
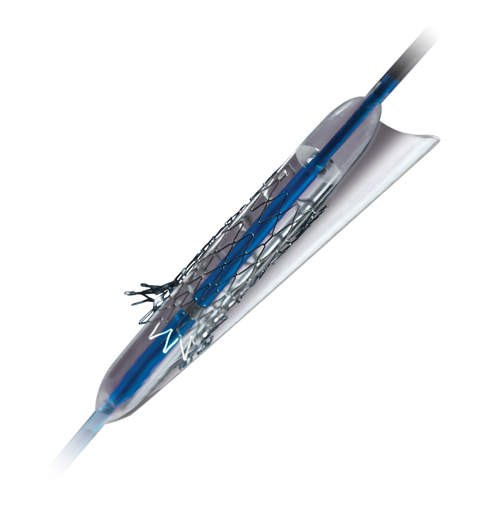
Coronary sidebranch stent and delivery system*
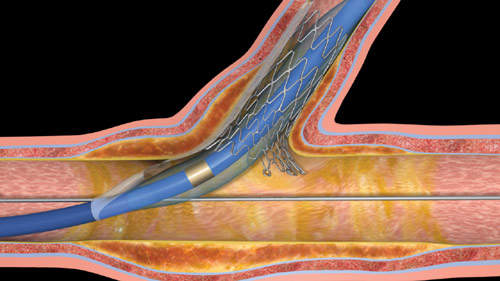
Sideguard’s novel, low-profile delivery system enables straightforward, rapid-exchange delivery and precise stent placement at the sidebranch ostium. The proprietary split-sheath technology prevents the nitinol stent jumping at delivery and ensures accurate deployment.
The markers are aligned at the sidebranch ostium. The delivery system balloon is then inflated, which splits the sheath and releases the nitinol stent. No special manipulations (rotation or alignment) are required prior to deployment. The delivery system is then withdrawn.
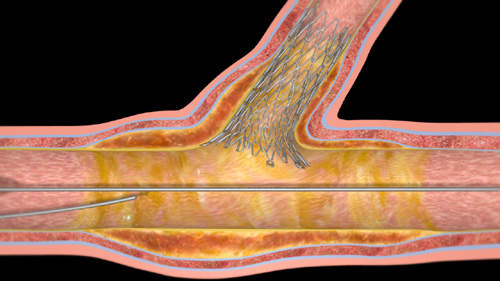
The self-expanding nitinol design conforms to the anatomy of the sidebranch ostium while the mechanical properties of nitinol sustain continuous wall apposition and scaffolding.
Dynamic apposition with the Sideguard coronary sidebranch stent
The treatment of bifurcation disease is a challenging procedure which, combined with the variability of the sidebranch ostium anatomy, requires a unique solution and an optimal stent design. Moreover, the structures requiring scaffolding are asymmetric and therefore the design must be self-adjusting. The elastic properties of nitinol allow such adaptability to various morphologies. The combination, therefore, of self-expanding nitinol with a unique, conforming stent design provides for constant outward radial force on the lumen.
The Sideguard stent therefore dynamically apposes the full sidebranch lumen including the ostium. This dynamic apposition results in continuous stent-to-vessel-wall contact as well as an increase in vessel diameter over time.
The advantages of the Sideguard coronary sidebranch stent design
The Sideguard stent is formed of three parts: the trumpet conforms to the sidebranch ostium; the gimbal provides expansion force to open the sidebranch; and the anchor is designed to prevent stent migration. This dynamic stent design ensures continuous wall apposition and positive remodelling.
The stent offers a dynamic treatment for bifurcation disease and used in combination with the low-profile balloon-catheter delivery system, it eliminates stent snagging through tortuous anatomies and calcified lesions.
R&D and manufacturing of sidebranch stents
Cappella Medical Devices, based in Galway, Ireland, is the R&D and manufacturing subsidiary of the Cappella group.
* Products not currently available for sale in the US.