Widespread predictions see the medical device market on a growth trend of about 15% over the next two years with innovations in pacemakers, defibrillators and nanotechnology leading the pack. Although the global economic downturn and rumours of a proposed tax on US medical device manufacturers – which could cost the industry up to $20bn over the next ten years – have put a slight dent on the industry’s confidence, new technologies still made their way on to the market during 2009. Here, we pick out five of the best.
Stomach pacemakers
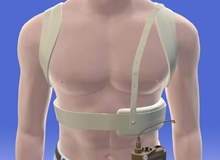
Implantable pacemakers have been around since the 1960s, regulating the heart and being used to treat adults with delayed gastric emptying. Now this technology has been adapted to help children with chronic stomach problems.
Sixteen-year-old Emma Geiger, along with over a million others, has suffered from gastroparesis for years. It is a condition that causes the stomach to contract less powerfully and as a result, capture food and liquids in the stomach, causing bloating, nausea, malnourishment and weight loss. So intrusive were the symptoms that eventually Emma had to leave school and study online instead.
When Emma was treated at the Nationwide Children’s Hospital in Columbus, Ohio, US in 2009, surgeons implanted a pacemaker inside her abdomen, which sends electrical impulses to stimulate the stomach after eating.
“The pacemaker is surgically implanted under the skin and is connected to two electrodes placed on the stomach wall,” explains paediatric surgeon Steven Teich, surgical director of the Bariatric Surgery Program at Nationwide Children’s Hospital and clinical associate professor of surgery at The Ohio State University College of Medicine.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData“It tells the stomach to empty at a certain frequency. The initial settings are fairly low and, as with a pacemaker in the heart, we can change the settings as needed.”
This successful surgery has shown that stomach pacemakers alleviate all symptoms in a very short period of time. “In patients who have received this type of treatment, nearly all symptoms were resolved within two weeks,” says paediatric gastroenterologist Hayat Mousa, medical director of the Motility Center at Nationwide Children’s Hospital and associate professor of clinical paediatrics at The Ohio State University College of Medicine.
The wearable defibrillator
August 2009 was a good month for US firm Zoll as it picked up Frost & Sullivan’s North American Healthcare Innovation Award for LifeVest – the world’s first wearable cardiac defibrillator.
This lightweight product is for patients at risk of sudden cardiac arrest (SCA) and consists of two components: a garment and a monitor. It helps protect them while their condition is changing and before a permanent SCA risk has been diagnosed.
Health experts estimate that SCA kills more than 400,000 Americans each year. Almost three quarters of victims defibrillated within a minute or two of survive; however, with each passing minute the chances of survival drop by 10%. When the LifeVest detects a life-threatening rhythm the device delivers a shock to restore a normal heart rhythm, in less than a minute.
The monitor collects ECG data and dry therapeutic electrodes automatically deploy conductive gel prior to a shock. It also alerts bystanders, but has a 98% first shock success rate for treating patients where intervention has not been required.
The LifeVest is used for patients following heart attack, before or after bypass surgery and those with cardiomyopathy or congestive heart failure. To date more than 20,000 patients have been prescribed the LifeVest.
A body-cooling suit
Medical Design Excellence Award winner of 2009, the ThermoSuit System (TSS) from Life Recovery Systems (LRS), is a rapid non-invasive patient cooling device that reduces the risk of brain or heart damage. The company’s founders Robert Freedman Jr and Dr Robert Schock developed the product after realising the existing treatment was inadequate and that rapid cooling could improve outcomes of critically ill patients.
The TSS is designed to be easily and quickly applied by nursing staff and can cool the core temperature of a cardiac arrest, stroke or heart attack victim to 32–34°C in 30 minutes or less. Once cooled the vital organs operate more slowly and need less oxygen, which reduces the chance of brain or heat damage.
Strong clinical results, presented at the American Heart Association Scientific Sessions, demonstrated the positive impact that TSS will have on patients. “We are very happy to see such favourable clinical results in the centres using the ThermoSuit System so far,” says Dr Schock. “This is a fulfillment of the mission Dr Freedman and I have had since founding Life Recovery Systems.”
Robotic snake
While brainstorming during a project focusing on using snake robotics for search and rescue missions, Dr Alon Wolf and Professor Howie Choset came up an idea that could reduce the need for ‘open’ surgery forever. They realised that if snakes could be sent inside buildings to look for survivors they could also be sent inside the body to perform minimally-invasive cardiac surgery, which prompted them to set up medical device firm Cardiorobotics.
There are more than 1 million cardiac procedures and more than 10 million relevant endoscopic and laparoscopic procedures performed each year in the US.
At the centre of the robotic snake technology is a tele-operated probe, which is flexible in shape. It requires single-port access for deep anatomical procedures and consists of a series of links, which can be made from any material, including plastic, allowing it to be disposable.
The benefits to patients when open surgery doesn’t have to be performed are enormous and include fewer incisions and injuries to nerves and major blood vessels, reduced blood loss and decreased pain. This means the patient will also experience a quicker return to normal activity.
The wireless plaster
Toumaz Technology, a provider of low-power wireless body monitoring devices, has started trials at St Mary’s Hospital in London on its Sensium digital plaster – a revolutionary device that records patient data wirelessly. This device looks like an ordinary plaster but is placed on the chest to wirelessly send patient data in real time to PDAs, mobile devices or hospital networks.
It replaces the need for expensive bedside monitoring equipment and means medical staff are constantly aware of the patient’s condition.
“This technology has the potential to improve the capturing of a patient’s vital signs within all areas of the hospital – enabling key physiological data to be acquired at an increased frequency, with the minimum of inconvenience to patients, and without the requirement to connect patients to immobile pieces of equipment,” says Dr Stephen Brett, an intensive care consultant at Imperial College Healthcare NHS Trust, who is leading the
trial.
“This raises the possibility of technology: improving hospital safety systems; enhancing the efficiency of adding vital sign data to patient records: and potentially freeing valuable nursing staff time for other patient care responsibilities.”
Toumaz’s Sensium range was named emerging technology company of the year by the National Microelectronics Institute (NMI). The device will be deployed into households once it has been proved in the hospital environment.







