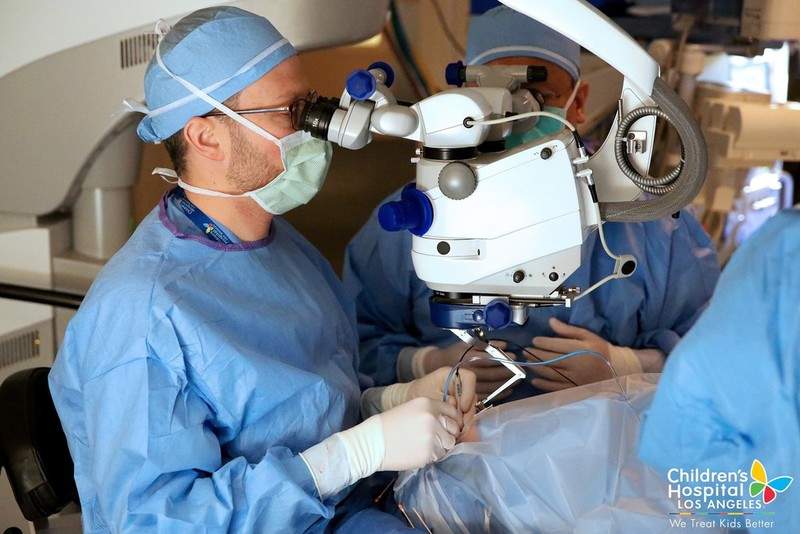
A team of surgeons at Children’s Hospital Los Angeles’ (CHLA) The Vision Center has successfully performed a gene replacement procedure, to restore vision in a patient with retinal degeneration.
The new one-time gene therapy replaces a defective gene known as RPE65 in the retina, the membrane at the back of the eye that detects light and colour, with a healthy replica produced using artificial DNA, the equivalent of human DNA.
RPE65 produces a protein that makes light receptors work in the eye.
CHLA The Vision Center director Thomas Lee said: “We are pleased to be able to offer this therapy that can truly impact a patient’s quality of life and, potentially, help them see their future through ‘new eyes’.”
Marketed as Luxturna, the therapy was developed by Spark Therepeutics and approved by the US Food and Drug Administration (FDA) last year to treat a rare, inherited form of blindness in both children and adults.
During a trial, it was found that the therapy helped restore vision for people between the ages of four and 44 with Leber congenital amaurosis (LCA) caused by RPE65 mutations.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataAfter treatment, the subjects were able to identify faces and could read for the first time in years.
Administered through a surgical procedure, the therapy allows the patients to go home later that same day after completion of surgery. After one week, the patient returns to get the surgery completed on his second eye.