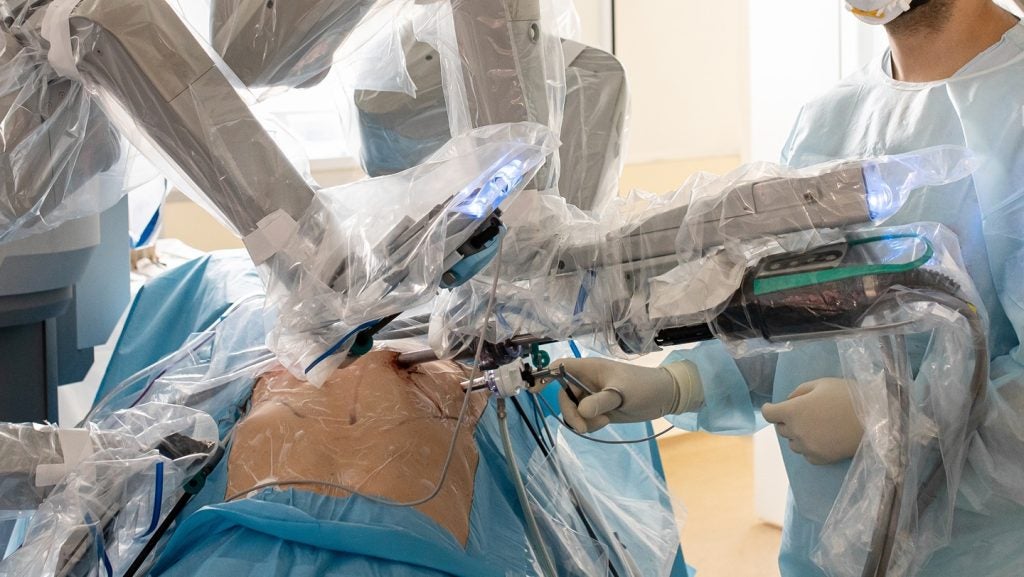
Emory University Hospital in Atlanta, US, has implemented Microbot Medical’s Liberty Endovascular Robotic System for patient care.
Microbot is also partnering with the hospital for establishing an endovascular robotics programme within the interventional radiology division.
Liberty is a single-use remotely operated robotic system for peripheral endovascular procedures and has received clearance from the US Food and Drug Administration (FDA).
The system is intended to enable accurate vascular navigation while seeking to reduce physical strain and radiation exposure for clinicians.
Microbot Medical recently initiated a limited market release of Liberty and intends to proceed with a full commercial launch at the Society of Interventional Radiology (SIR) conference scheduled for April 2026.
Emory University Hospital will use the Liberty system to support clinicians in peripheral intervention procedures such as uterine fibroid embolisation, liver tumour embolisation, and prostate artery embolisation.
The collaboration aims to enhance care delivery in oncology and endovascular services.
Emory University School of Medicine's interventional radiology division director David Prologo said: “Introducing innovative technologies such as Liberty to our interventional radiology programme underscores our priority to remain at the forefront of technological advancement.
“We anticipate that its single-use, remotely operated design will offer a practical and scalable approach to robotics that supports our operational goals, protects our clinicians and provides access to quality care.”
Microbot Medical CEO, president and chairman Harel Gadot said: “This is an extraordinary occasion for Microbot Medical, and we believe a major milestone for the entire surgical robotic space.
“We are establishing a completely new medical robotic category with the adoption of the first FDA-cleared single-use robotic system.”